You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Water readings
- Thread starter TofferMora
- Start date
The April FOTM Contest Poll is open!

🏆 Click to vote! 🏆
For aquarists, the primary water issues are the hardness (GH is this value, which is basically the level of dissolved calcium and magnesium), the pH, and the KH (carbonate hardness also called Alkalinity). These three are related, and usually match: a high GH tends to mean a similarly high KH, or a low GH a lower KH. The GH and (especially) KH serve to "buffer" the pH. Sometimes other issues affect the pH, such as dissolved CO2 in the water, substances the water authority may add to increase pH, etc. I'll come back to these.
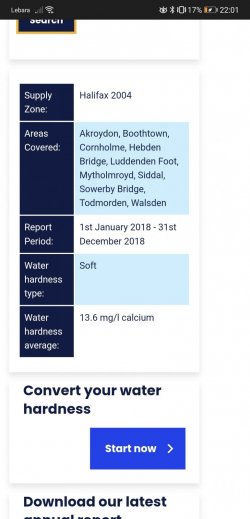
The other important aspects for aquarists are the nitrogen numbers, meaning the ammonia, nitrite and nitrate. We can dismiss these fairly quickly, as they are not problematic. Ammonia is not given (you could test this yourself), nitrite at 0.002 mg/l is not a problem, and nitrate at 1.86 mg/l is certainly not an issue. I will just mention that the hobby uses ppm which is the same value as mg/l when testing/measuring ammonia, nitrite and nitrate.
Back to the GH/pH. At 13.6 mg/l (equates to 13.6 ppm) you have very soft water. The KH is not given (common in the UK I believe, @Essjay can confirm or advise) but I would expect it is similarly low. This means the pH will be more likely to lower as the aquarium establishes. As for the pH in the tap water, you need to out-gas any dissolved CO2 before testing; the easiest way is to let a glass of water sit 24 hours, then test. That will be the more accurate reading (and may be higher, depending upon the CO2). Once you pin this down, do not use both the basic and the high pH tests, as they will always be different. If the pH turns out to be below 7.6 then use the basic. If it turns out to be around 8, use the high range. The source water pH is the thing that determines which test. The numbers here for pH indicate 7.6 so you will be using the basic test unless we are missing something.
The other important aspects for aquarists are the nitrogen numbers, meaning the ammonia, nitrite and nitrate. We can dismiss these fairly quickly, as they are not problematic. Ammonia is not given (you could test this yourself), nitrite at 0.002 mg/l is not a problem, and nitrate at 1.86 mg/l is certainly not an issue. I will just mention that the hobby uses ppm which is the same value as mg/l when testing/measuring ammonia, nitrite and nitrate.
Back to the GH/pH. At 13.6 mg/l (equates to 13.6 ppm) you have very soft water. The KH is not given (common in the UK I believe, @Essjay can confirm or advise) but I would expect it is similarly low. This means the pH will be more likely to lower as the aquarium establishes. As for the pH in the tap water, you need to out-gas any dissolved CO2 before testing; the easiest way is to let a glass of water sit 24 hours, then test. That will be the more accurate reading (and may be higher, depending upon the CO2). Once you pin this down, do not use both the basic and the high pH tests, as they will always be different. If the pH turns out to be below 7.6 then use the basic. If it turns out to be around 8, use the high range. The source water pH is the thing that determines which test. The numbers here for pH indicate 7.6 so you will be using the basic test unless we are missing something.
TofferMora
Fish Fanatic
Ok thanks very much for all the info.
As Byron said, UK water companies rarely list KH - if they do they call it alkalinity. KH is less important to fish than GH, that's the one we really need to know.
Latest Discussions
- Replies
- 4
- Views
- 29