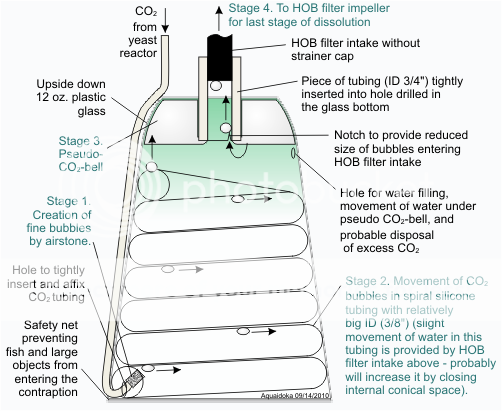
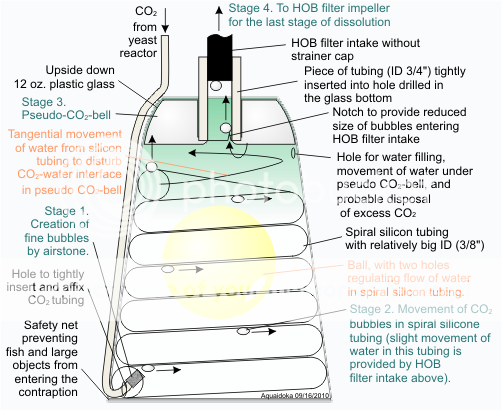
aquaidoka
New Member
Thanks... The main difficulty in this kind of learning is that many pros provide contradictory information. Of course, I would prefer heavily peer-reviewed literature, but there isn't a lot of time for this kind of research. Well, I can imagine that nutrients together with water and air are friends of all living beings when used moderately, and heard that algae are not quite the enemies of the aquarium but one of the indicators of good conditions. There is no doubt, that being the living beings, algae do need the nutrients (not the same ones as those needed by fish, but rather produced by fish, plants other wastes). So it may be not correct to say that when algae lack their nutrients, they thrive. Of course if everything will start to die and rot in the aquarium because there would be a lack of nutrients for plants and fish, the algae would bloom for some time because of their food appearance. But it is hard to call as a "lack" of nutrients. So, I'll continue to think that nutrients (for algae) should be removed/should not be added to overcome bloom of algae.
Concerning CO2, usually people write that 3 W/Gal is a critical amount of light when you can decide whether to use CO2 or not. As a chemist, I feel an internal resistance to the addition of CO2 to aquarium with alive fish, which can be harmful for them if proper addition is not guaranteed (if used compressed in cylinder - potentially dangerous to my family). On the other hand, as a beginner of fishkeeping, I would really like to have a lot of plants. So I decided to use for now the amount of light, which does not critically demand addition of CO2 yet, and plants, for which CO2 addition is not necessary, and minimum amount of needed light is 2 W/Gal. Well, I can reduce the light to 2.4 W/Gal, and sit down and think about the proper use of CO2 probably produced by yeast. Again about the time of using the lights, only on these pages we can read about the recommended proper use of 8 to 12 hour (and more if needed for algae growth). In this case, I think, I should come to the correct answer by experiment and observation. 10 Hours show positive dynamics of clarification in my case for now.
Does somebody have answers for the aeration questions above?
Concerning CO2, usually people write that 3 W/Gal is a critical amount of light when you can decide whether to use CO2 or not. As a chemist, I feel an internal resistance to the addition of CO2 to aquarium with alive fish, which can be harmful for them if proper addition is not guaranteed (if used compressed in cylinder - potentially dangerous to my family). On the other hand, as a beginner of fishkeeping, I would really like to have a lot of plants. So I decided to use for now the amount of light, which does not critically demand addition of CO2 yet, and plants, for which CO2 addition is not necessary, and minimum amount of needed light is 2 W/Gal. Well, I can reduce the light to 2.4 W/Gal, and sit down and think about the proper use of CO2 probably produced by yeast. Again about the time of using the lights, only on these pages we can read about the recommended proper use of 8 to 12 hour (and more if needed for algae growth). In this case, I think, I should come to the correct answer by experiment and observation. 10 Hours show positive dynamics of clarification in my case for now.
Does somebody have answers for the aeration questions above?