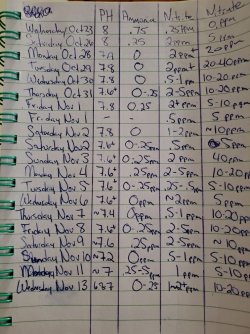
Hey all,
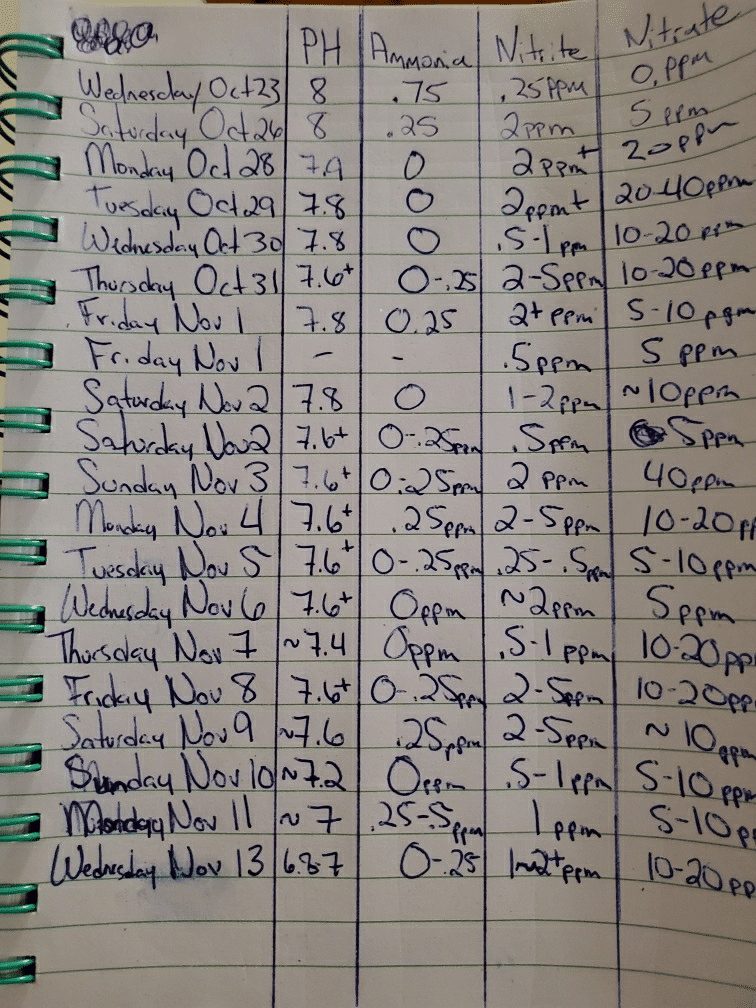
We're beginning to run into a new problem. Over the past several weeks our ph has been slowly dropping, to where now it's about 6.8. A few weeks ago it was holding steady at about 7.6-7.8. I know that our fish prefer the lower ph so it isn't an issue at the moment, but we don't know what's causing it to drop and how far it's going to go. I don't want it to drop too far below 6.6 or so. I've included our water parameter chart that we've been keeping so you can see our levels and fluctuations. (If you haven't read my other posts, we added fish a little too early and are still in the last stages of the cycle, that is why the high nitrite readings. We're doing water changes every 2-3 days and dousing with Prime a couple of times a day.)
What we have in our tank:
Inert gravel substrate
One medium sized piece of driftwood (~12in high x~6-7in wide)
One medium sized lava stone (~10in high x ~6in wide)
Two halves of a ship decoration
Three fake plants
Two Anubias plants
Four moss balls
6 Blue Moscow Guppies with about 15-20 fry
2 Bristlenose Plecos
3 Yoyo Loaches
Does anyone know if any of the decorations we have would bring down the ph? Right now I'm looking at the driftwood and the plants as the culprit, or is it just a natural part of the cycle for the ph to go down? Also if it keeps going down, what can we do to get it back to 6.5-7?
We're beginning to run into a new problem. Over the past several weeks our ph has been slowly dropping, to where now it's about 6.8. A few weeks ago it was holding steady at about 7.6-7.8. I know that our fish prefer the lower ph so it isn't an issue at the moment, but we don't know what's causing it to drop and how far it's going to go. I don't want it to drop too far below 6.6 or so. I've included our water parameter chart that we've been keeping so you can see our levels and fluctuations. (If you haven't read my other posts, we added fish a little too early and are still in the last stages of the cycle, that is why the high nitrite readings. We're doing water changes every 2-3 days and dousing with Prime a couple of times a day.)
What we have in our tank:
Inert gravel substrate
One medium sized piece of driftwood (~12in high x~6-7in wide)
One medium sized lava stone (~10in high x ~6in wide)
Two halves of a ship decoration
Three fake plants
Two Anubias plants
Four moss balls
6 Blue Moscow Guppies with about 15-20 fry
2 Bristlenose Plecos
3 Yoyo Loaches
Does anyone know if any of the decorations we have would bring down the ph? Right now I'm looking at the driftwood and the plants as the culprit, or is it just a natural part of the cycle for the ph to go down? Also if it keeps going down, what can we do to get it back to 6.5-7?