hobby- in pH 5 water there is 0 NH3 and 100% NH4. So the question you need to answer for me is simple. I have a tank with wild fish. I keep the pH in the mid 4s. I test for ammonia and learn I have .5 ppm. I know there is no NH3. How long can the fish live in that .5 ppm of NH4 and suffer 0 harm or damage? Suppose that level doubles? How about if its 4 ppm, all NH4. Can you answer this for me?
There is more to needing the pH kept up in nitrification than simply the pH and the acid factor. I can show you research that has autotrophic nitrification happening in pH 4.0. The Nitrosomonas europea strain studied in it have receptors for NH4 which allows them to utilize it. There is also the fact that the dropping of pH usually happens because the carbonates in KH get used up. And they are usually a big source of the inorganic carbon for the bacteria. Remove that and they can't oxidize.
Hobby- I have been studying this topic in depth for several years. I do understand the nature of NH3 and NH4. I have not been trapped by anything. But you are making a statement here on which most of the membership will challenge you as they have me. In testing ammonia we get results for both forms. Most folks stop here and determine if that reading is harmful and how harmful based on the combined total ammonia reading. I have been arguing for some time now that this is not the proper approach.
You are not quite correct in what you state about research related to aquariums. While is it not an overriding issue, it is also not one where there is nothing to be found.:
Comparative Analysis of Nitrifying Bacteria Associated with Freshwater and Marine Aquaria. Applied and Environmental Microbiology Vol. 62, No. 8: 2888-2896. Hovanec, T. A. and E. F. DeLong. 1996.
http /aem.asm.org/content/62/8/2888.full.pdf+html
/aem.asm.org/content/62/8/2888.full.pdf+html
Nitrospira- Like Bacteria Associated with Nitrite Oxidation in Freshwater Aquaria. Applied and Environmental Microbiology Vol. 64, No. 1: 258-264. Hovanec, T. A., L. T. Taylor, A. Blakis and E. F. DeLong. 1998.
http /aem.asm.org/content/64/1/258.full
/aem.asm.org/content/64/1/258.full
Identification of Bacteria Responsible for Ammonia Oxidation in Freshwater Aquaria. Applied and Environmental Microbiology, Dec. 2001, p. 5791-5800. Paul C. Burrell, Carol M. Phalen, and Timothy A. Hovanec.
http /aem.asm.org/content/67/12/5791.full
/aem.asm.org/content/67/12/5791.full
And there are other studies that are related to aquariums. However, there are many more related to aquaculture and drinking water treatment. Here the same nitrifying bacteria are at work but now there is lots of impetus behind and funding for research. And we can extrapolate a lot of this to aquariums.
I have read countless abstracts or full studies on this topic, every one looks directly at NH3. I have seen studies for specific fish, for chronic exposure and for the effects of high ammonia and nitrite. I have read a Ph.D. thesis or two on the bacteria and biofilms and chlorine and chloramine. Trust me, I am not confused. I have been using ammonium chloride for some time to produce NH3 in tanks. What I am trying to do is explain to fish keepers that when it comes to ammonia, most fish sites have it all wrong. My hope was I could make this hit home if dechlors basically bound ammonia in the form of ammonium and prevented the interchange for some period. It would mean the fish were still exposed to the NH4 but not harmed. And this for me was a backdoor approach. If the product detoxified ammonia making it non-harmful and it did this as I just described, it would make it very hard for the doubters to continue doing so. Unfortunately, I struck out.
My error was in how I stated the NH3 - NH4 relationship. I know the two balance based on the pH and temp. However, I was trying to make the point that in taking up all the ammonia in a tank the bacteria only used the NH3 part. Because the bacteria will not process all the ammonia in a tank until they are present in sufficient numbers, they will take up only a part. They need to use up what they have taken in before they take in more. I was trying to say they will never use it all up in one fast go until the cycle is established, they are removing it in increments. What happens is they take it in smaller steps along the way. This lowers total ammonia, but it happens in steps. I stated this poorly (in terms of the actual chemical process) in my number of steps illustration.
There is a difference between how ammonia is produced and handled in a cycled tank vs how it is processed as it is added during a fishless cycle. My error was in how I tried to illustrate this.
However, much of what you noted about ammonia toxicity I have been saying for a long time on this site and others. I am not the one you need to be explaining this too, rather it is most of the rest of the membership here.
But as a microbiologist perhaps you can clarify something for me. The way the detoxifiers work is by changing the structue of the NH3 (and I presume the NH4 as well?). They substitue some number of the Hs with other things like this:
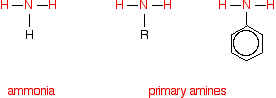
Comparing the structures of ammonia and primary amines
Each substance contains an -NH2 group. In ammonia, this is attached to a hydrogen atom. In a primary amine, it is attached to an alkyl group (shown by "R" in the diagram below) or a benzene ring.
from
http /www.chemguide.co.uk/organicprops/anhydrides/nitrogen.html
/www.chemguide.co.uk/organicprops/anhydrides/nitrogen.html
What I was having difficulty understaning in relation to the bacteria realtes to what I wrote about the N being present all the way through but was changing its charge. My understanding is that the process was essential in a chain of fixing nitrogen. I believe what the bacteria are doing is helping to fix N in a form that can readily be consumed up the chain but that the bacteria were not consuming the N themselves. Plants love nitrate but can not use N directly, animals eat plants but do not use N directly. Have I understood this wrong? What I was trying to disover was how the process would still work on the bacterial level if the some of the H components were altered. First I wondered how the bacteria could take in the altered form and, second, if they did, how the process would still work in the absence of one of more of the Hs in NH3/NH4.
Finally, I agree with you that the average fish keeper doesn't have a clue about most of this and should never need to have one. However, I also believe that if one is going to opt for doing a fish in cycle, then they do need to know about the NH3/NH4 facts, they need to know how to do diluted nitrite testing, they need to know how to counteract nitrite with chloride. If they don't wish to have to learn and master so much more and to dedicate so much more work and time to cycling, they should simply be doing a fishless cycle. If they follow the directions in the cycling article on this site (which I wrote), they will cycle a tank in good time and with few problems. And they do not have to know anything about NH3/NH4 or how to test nitrite that is over 5 ppm on their API kits. But when it comes to fish in cycling, what they should not be doing is a lot of unneeded water changes which can also be harmful in terms of fish stress levels.


 /louisville.edu/research/for-faculty-staff/reference-search/2010-medicine/2004-references/arimitsu-et-al-2004-synthesis-of-a-stable-imidium-complex-derived-from-gamma-silyl-alpha-alpha-difluorobromopropyne-evaluation-of-aja-experimental-parameters
/louisville.edu/research/for-faculty-staff/reference-search/2010-medicine/2004-references/arimitsu-et-al-2004-synthesis-of-a-stable-imidium-complex-derived-from-gamma-silyl-alpha-alpha-difluorobromopropyne-evaluation-of-aja-experimental-parameters