55 gallon, 7.5-8.5 ph, idk the kh or gh. Yes I am wanting a Strict Biotope. Why is kh and gh important anyways?
I will answer by citing excerpts from an article on water hardness I wrote a few years ago on another forum. This will provide a detailed explanation, but it doesn't hurt to know the whole story.
Water in its pure form does not exist in nature (aside from the evaporated and condensed pure water before it rains/snows); it is a powerful solvent, meaning a substance that easily dissolves other substances to create a solution. As rain falls, it picks up many gasses and particulate matter, and it continues to do this as it passes through the ground. Natural water values therefore vary with respect to hardness and pH because the water acquires specific properties from the landscape. Water flowing over or through rock will assimilate minerals from the rock, becoming what we term “hard” water. Water flowing through soils that contain organic matter will be “soft” because the organics bind with and thus remove minerals while creating acids that enter the water. The pH is largely the result of the hardness as well as the amount of carbon dioxide dissolved in the water.
Water hardness is the measure of dissolved mineral salts in the water, a portion of the TDS (total dissolved solids). There are two basic types of hardness of importance to aquarists, termed general hardness (abbreviated GH) and carbonate hardness (abbreviated KH, from the German “karbon” [carbon]). The combined GH and KH is sometimes termed “total hardness,” but this is of less importance because the GH and KH individually impact the water in different ways.
General Hardness is determined primarily by the minerals calcium and magnesium; GH is sometimes referred to as “permanent hardness” because it cannot be removed from water by boiling as can KH. GH is measured in several different units, but in the hobby the most common are parts per million (ppm) and degrees (dH or dGH). One dGH equals 10 milligrams of calcium or magnesium oxide per litre [1], and is equivalent to 17.848 ppm. Multiplying dGH by 17.9 gives ppm, and similarly dividing ppm by 17.9 gives dGH [the same formula works for KH]. The following chart equates the degrees and relative ppm to common terms in the hobby.
0 - 4 dGH 0 - 70 ppm very soft
4 - 8 dGH 70 - 140 ppm soft
8 - 12 dGH 140 - 210 ppm medium hard
12 - 18 dGH 210 - 320 ppm fairly hard
18 - 30 dGH 320 - 530 ppm hard
over 30 dGH over 530 ppm very hard
As each freshwater fish species has evolved over thousands of years, their physiology has adjusted to the water values that occur in their respective habitat. We refer to these values as water parameters, and they include hardness, pH and temperature; each of these has an impact on fish. While many fish species appear to be somewhat adaptable, their physiology can be negatively affected if the parameters are outside the fish’s natural preference. Providing suitable water parameters in the aquarium is therefore an important aspect of providing an environment that is less stressful—and this directly relates to healthier fish.
Fish are directly impacted by GH and TDS; their growth, the transfer of nutrients and waste products through cell membranes, spawning (sperm transfer, egg fertility or hatching), and the proper functioning of internal organs such as the kidneys can all be affected.
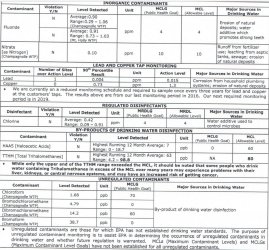
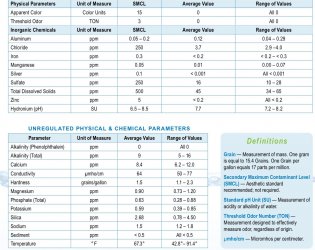
Rather than buying a test kit you may only use the once, check the website of your municipal water authority; it may have data including GH and KH. The GH is the most critical here. Fish that need moderately hard or harder water must have this or they cannot function properly (livebearers, rift lake cichlids, some rainbowfish, and a few others as an example). Soft water species (most fish from South America and SE Asia in general) need softer water. There is some overlap obviously, depending upon species and the water parameters.