NOTE: I actually did this research prior to spotting the thread in the beginner's section of the forum titled "Toxicity of Ammonia". After comparing my research to the results posted there, I am getting different "factor" results than the percentages posted in that thread. I'm going to lay out my research here, and then perhaps we can discuss why those numbers are different from these. I verified the numbers I used from three different research papers. (bibliography provided at the end) I may have misunderstood how the numbers in the other article are being used.
BACKGROUND
I went down this road because I have very soft aquarium water with a pH of 6.0, and whenever I carefully clean my filters, or take media out to put in another tank, I enter a "mini-cycle" that typically lasts from 3 to 4 weeks. The bacteria colonies recover more slowly in the low pH. The ammonia will usually register 0.25 to 0.50 ppm during the recovery period, and water changes do very little to help, since my tap water measures 0.25 ppm of ammonia. It is very frustrating, and I began researching to see just how toxic this level of ammonia is to my fish. It turns out I did not have as much to worry about as I thought.
ANY LEVEL OF AMMONIA IS BAD
It is important to note that ANY level of ammonia indicates an imbalance in the nitrogen cycle, and you should take whatever steps are needed to correct the situation as quickly as possible. But using the information in this article can help you determine whether you are in an emergency situation (actively harming the fish), or if you can take corrective action less urgently.
EDIT: After corresponding with some senior members on the forum, it should also be noted that fish sensitivity to ammonia can vary greatly by species. So use the 0.05 toxicity point with great caution. You might have fish that are more sensitive than that.
AMMONIA BREAKDOWN
The ammonia measured by your test kit actually gives you one total value containing two different forms of ammonia. This total is known as the Total Ammonia Nitrogen, or TAN.
The TAN is made up of:
1. NH4+: Ionized ammonia (ammonium): so named because it has a positive charge.
2. NH3: Un-ionized ammonia: has no charge.
Of the two ammonia types, NH3 is BY FAR more dangerous to your fish. Any level of NH3 greater than 0.05 ppm is considered to be a toxic level for fish, and a level of NH3 of 2.0 will kill your fish (Univ. of Florida, "Ammonia in Aquatic Systems"). The level of NH3 versus NH4+ will vary based on your pH level and your temperature. At higher levels of pH and temperature you will find higher (and more toxic) amounts of NH3 ammonia.
HOW TO CALCULATE THE LEVEL OF NH3 AMMONIA
Several research studies have already created reference tables containing the factors used to multiply your TAN reading to calculate the level of NH3. I took those tables and selected what I thought would be the most common range of temperature and pH used for freshwater aquariums. Here is a print screen of the table, but if you would also like the original Excel spreadsheet, just send me a message.
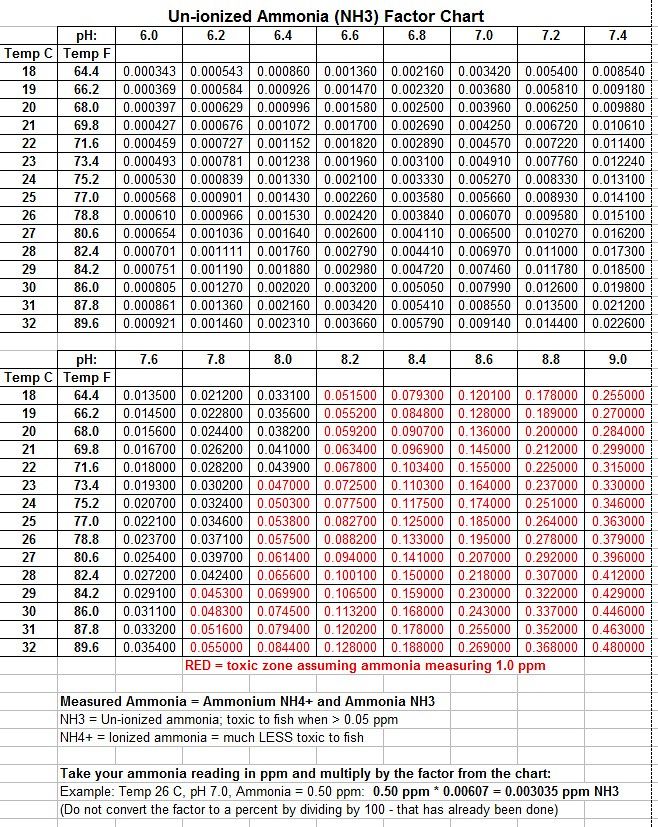
FORMULA: NH3 = TAN * Factor
TAN= the ppm total of ammonia from your tested tank water
Factor = the decimal number from the chart corresponding to your cross referenced pH and temperature
The research studies I looked at had the factors stated as percentages, requiring you to divide by 100 before multiplying by the TAN. But I divided by 100 prior to putting the numbers into the spreadsheet, so you do not have to do that. Just take your TAN and multiply by the number from the table.
For my own tank reading, my TAN was 0.50 ppm ammonia, with a pH of 6.0, and temperature of 26C. The factor table gave me a conversion factor of 0.000610:
0.50 ppm * 0.000610 = 0.000305 ppm NH3
This value is WELL below the toxic level for ammonia, so I know that my fish are not in serious danger while the tank recovers from the mini-cycle.
If you are in a situation where you are waiting out a mini-cycle, and you cannot dilute the ammonia with water changes, you should at least closely monitor your pH and calculate your level of NH3 to ensure your fish are not suffering from toxic ammonia levels.
BIBLIOGRAPHY
Florida Department of Environmental Protection, Tallahassee, FL. "Calculation of Un-ionized Ammonia in Fresh Water, STORET Parameter Code 00619". Revision 2: Feb 12, 2001.
Francis-Floyd, Ruth, Craig Watson, Denise Petty, and Deborah B. Pouder. "Ammonia in Aquatic Systems". University of Florida IFAS Extension; first published: May 1990; revisions: June, 1996, February 2009.
Griffitts, Tony. "Ammonia Toxicity and the pH Relationship". "Aquaworld Aquarium" online fish magazine.
Thurston, Robert V., Rosemarie C. Russo, and Kenneth Emerson. "Aqueous Ammonia Equilibrium - Tabulation of Percent of Un-Ionized Ammonia". Montana State University & The United States Environmental Protection Agency. August 1979.
BACKGROUND
I went down this road because I have very soft aquarium water with a pH of 6.0, and whenever I carefully clean my filters, or take media out to put in another tank, I enter a "mini-cycle" that typically lasts from 3 to 4 weeks. The bacteria colonies recover more slowly in the low pH. The ammonia will usually register 0.25 to 0.50 ppm during the recovery period, and water changes do very little to help, since my tap water measures 0.25 ppm of ammonia. It is very frustrating, and I began researching to see just how toxic this level of ammonia is to my fish. It turns out I did not have as much to worry about as I thought.
ANY LEVEL OF AMMONIA IS BAD
It is important to note that ANY level of ammonia indicates an imbalance in the nitrogen cycle, and you should take whatever steps are needed to correct the situation as quickly as possible. But using the information in this article can help you determine whether you are in an emergency situation (actively harming the fish), or if you can take corrective action less urgently.
EDIT: After corresponding with some senior members on the forum, it should also be noted that fish sensitivity to ammonia can vary greatly by species. So use the 0.05 toxicity point with great caution. You might have fish that are more sensitive than that.
AMMONIA BREAKDOWN
The ammonia measured by your test kit actually gives you one total value containing two different forms of ammonia. This total is known as the Total Ammonia Nitrogen, or TAN.
The TAN is made up of:
1. NH4+: Ionized ammonia (ammonium): so named because it has a positive charge.
2. NH3: Un-ionized ammonia: has no charge.
Of the two ammonia types, NH3 is BY FAR more dangerous to your fish. Any level of NH3 greater than 0.05 ppm is considered to be a toxic level for fish, and a level of NH3 of 2.0 will kill your fish (Univ. of Florida, "Ammonia in Aquatic Systems"). The level of NH3 versus NH4+ will vary based on your pH level and your temperature. At higher levels of pH and temperature you will find higher (and more toxic) amounts of NH3 ammonia.
HOW TO CALCULATE THE LEVEL OF NH3 AMMONIA
Several research studies have already created reference tables containing the factors used to multiply your TAN reading to calculate the level of NH3. I took those tables and selected what I thought would be the most common range of temperature and pH used for freshwater aquariums. Here is a print screen of the table, but if you would also like the original Excel spreadsheet, just send me a message.

FORMULA: NH3 = TAN * Factor
TAN= the ppm total of ammonia from your tested tank water
Factor = the decimal number from the chart corresponding to your cross referenced pH and temperature
The research studies I looked at had the factors stated as percentages, requiring you to divide by 100 before multiplying by the TAN. But I divided by 100 prior to putting the numbers into the spreadsheet, so you do not have to do that. Just take your TAN and multiply by the number from the table.
For my own tank reading, my TAN was 0.50 ppm ammonia, with a pH of 6.0, and temperature of 26C. The factor table gave me a conversion factor of 0.000610:
0.50 ppm * 0.000610 = 0.000305 ppm NH3
This value is WELL below the toxic level for ammonia, so I know that my fish are not in serious danger while the tank recovers from the mini-cycle.
If you are in a situation where you are waiting out a mini-cycle, and you cannot dilute the ammonia with water changes, you should at least closely monitor your pH and calculate your level of NH3 to ensure your fish are not suffering from toxic ammonia levels.
BIBLIOGRAPHY
Florida Department of Environmental Protection, Tallahassee, FL. "Calculation of Un-ionized Ammonia in Fresh Water, STORET Parameter Code 00619". Revision 2: Feb 12, 2001.
Francis-Floyd, Ruth, Craig Watson, Denise Petty, and Deborah B. Pouder. "Ammonia in Aquatic Systems". University of Florida IFAS Extension; first published: May 1990; revisions: June, 1996, February 2009.
Griffitts, Tony. "Ammonia Toxicity and the pH Relationship". "Aquaworld Aquarium" online fish magazine.
Thurston, Robert V., Rosemarie C. Russo, and Kenneth Emerson. "Aqueous Ammonia Equilibrium - Tabulation of Percent of Un-Ionized Ammonia". Montana State University & The United States Environmental Protection Agency. August 1979.


 /www.fishforums.net/index.php?/topic/154313-of-toxic-ammonia-charts/
/www.fishforums.net/index.php?/topic/154313-of-toxic-ammonia-charts/


 There are exceptions, I agree.
There are exceptions, I agree.

